





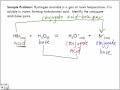












The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. The stronger an acid, the weaker its conjugate base, and, conversely, the stronger a base, the weaker its conjugate acid.. TABLE \(\PageIndex{1}\):Important Conjugate Acid-Base Pairs.. Table \(\PageIndex{1}\) gives a list of some of the more important conjugate acid-base Study guide Acids and Bases - Conjugate Pairs. This concept is examinable in the grade 12 exams for physical science in both the IEB and CAPS syllabus. Acid and bases conjugate pairs are introduced and explained in this document with examples. Particularly in the realm of complex numbers and irrational numbers, and more specifically when speaking of the roots of polynomials, a conjugate pair is a pair of numbers whose product is an expression of real integers and/or including variables. A complex number example: , a product of 13 An irrational example: , a product of 1. Or: , a product of -25. A simple buffer system might be a 0.2 M solution of sodium acetate; the conjugate pair here is acetic acid HAc and its conjugate base, the acetate ion Ac –. The idea is that this conjugate pair "pool" will be available to gobble up any small (≤ 10 –3 M) addition of H+ or OH – that may result from other processes going on in the solution. The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair. Some common examples of conjugate acid base pairs are, HClO 4 ⇆ H + + ClO 4–. H 2 SO 4 ⇆ H + + HSO 4–. HCl ⇆ H + + Cl –. Thus Cl – ion is a conjugate base of HCl and H 3 O + is the conjugate acid of base H 2 O. Such pairs of substances which differ from one another by a proton are known as conjugate acid-base pairs. Thus any acid-base reaction really involves two acids and two bases, forming conjugate pairs. Here is the one conjugate pair from the first example reaction: HCl and Cl¯. Usually, HCl is called an acid and Cl¯ is called its conjugate base, but that can be reversed if the context calls for it. So, we can correctly speak of Cl¯ as a base and HCl as its conjugate acid. Acid and bases conjugate pairs are introduced and explained in this document with examples. This concept is examinable in the grade 12 exams for physical science in both the IEB and CAPS syllabus. Acid and bases conjugate pairs are introduced and explained in this document with examples. Study guide - Quantitative chemistry - introduction We think of them in pairs, called conjugate pairs. When the acid, HA, loses a proton it forms a base, A-. When the base, A-, accepts a proton back again, it obviously refoms the acid, HA. These two are a conjugate pair. Members of a conjugate pair differ from each other by the presence or absence of the transferable hydrogen ion. Conjugate Pairs Practice Questions 1. Identify the acid, base, conjugate acid and conjugate base for each of the following. a) HClO 4 (aq) + H 2 O(l) ⇌ H 3
[index] [8742] [3914] [4837] [1672] [8522] [3682] [8963] [2066] [7738] [2514]
Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com https://www.iitutor.com Amphiprotic substances have the ability to behave as either B-L acids or B-L bases. Some examples of amphiprotic substances include H2O, HCO3-, HPO42-, HS-. If water is a ... Chemistry: Conjugate Acid-Base Pairs - Duration: 5:55. Pathways to Chemistry 4,563 views. 5:55 . What is Resonance ? Unit-4CBSE Class 11 Chemistry Resonance Structures - Duration: 10:44 ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Identifying Conjugate Acids-Base Pairs From a Chemical Equation 001 ... How to do conjugate acid and base pairs: Example conjugate problems - Duration: 10:09. MrHren 1,063 views. 10:09 . Language ... Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry - Duration: 11:37. ... (Henderson-Hasselbalch Example ) - Duration: 10:17. chemistNATE 46,967 views. 10:17 ... Acids and bases, conjugate acid base pairs Webinar for the New Normal: "Math Online Learning and Powerful Tools for your e - Classroom" Teacher Gon 242 watching Live now Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together...
Copyright © 2024 top100.realmoneytopgames.xyz